Bleach is a solution of a compound called sodium hypochlorite; sometimes calcium hypochlorite is called bleach as well. Both compounds dissolve readily in water and are highly alkaline. The Material Safety Data Sheet for Clorox regular bleach, for example, lists the pH of its product as 11.9. Chlorine bleach (a solution of approximately 3–6% sodium hypochlorite, NaClO) and ammonia, when used together, can have very harmful effects on the body. It is especially important to avoid exposing the skin or eyes to bleach when handling it, as such an exposure can cause severe tissue damage. Continue reading
Tag Archives: Carbamide peroxide
Teeth whitening with carbamide peroxide
What is carbamide peroxide in teeth whitening gels
Carbamide peroxide is also known as urea peroxide or urea hydrogen peroxide. Like any other peroxides, they are oxidizing agents which releases oxygen when in contact with water. The chemical formula is CH6N2O3, or CH4N2O.H2O2.
The carbamide peroxide is a skin, eye and respiratory irritant. It is also corrosive and causes burns. It does not cause injuries at 10% concentration but it might at 35% concentration hence causing white chemical burns on the skin and gums alike. Continue reading
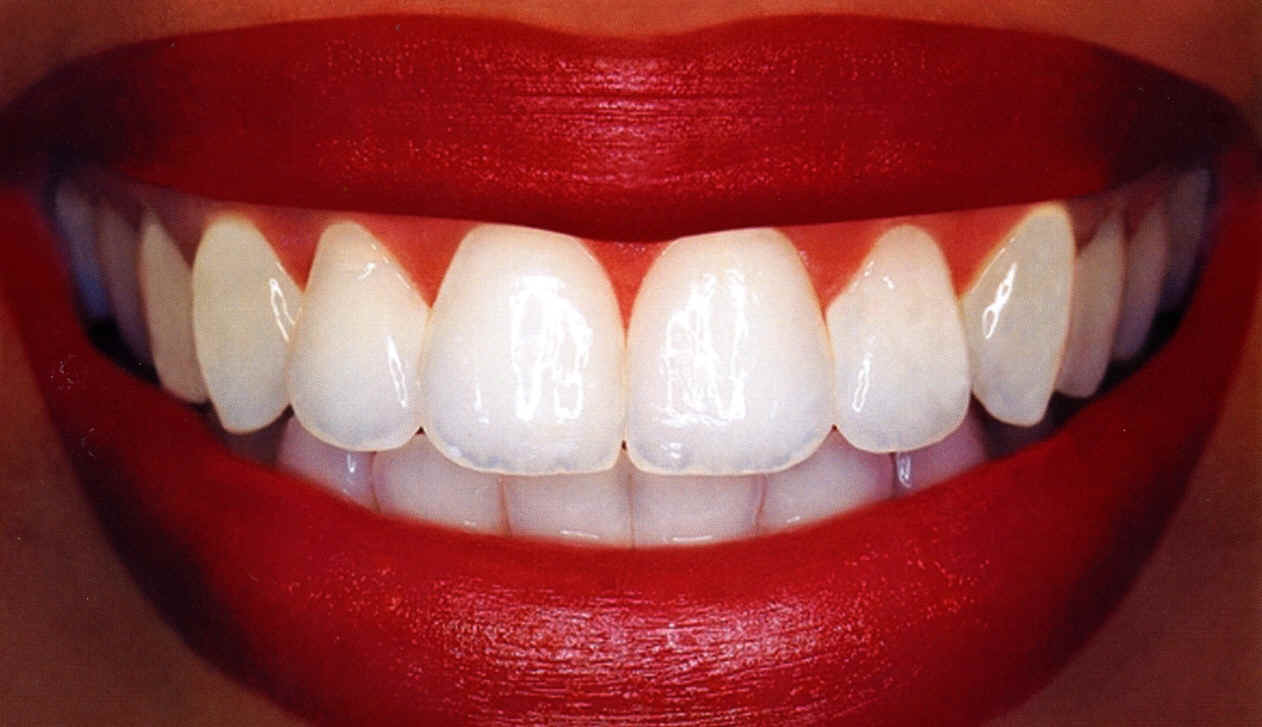
Teeth Whitening
TEETH WHITENING
Brighter teeth now are getting center stage in cosmetic dentistry. Most frequent reason patients seek for this dental care is discolored anterior teeth. Patients with normal teeth shades also do sometimes request for whitening treatments. Treatment options of discolored teeth include:
- Removal of surface stains
- Bleaching
- Microabrasions and/or macroabrasions
- Veneer
- Porcelain crown