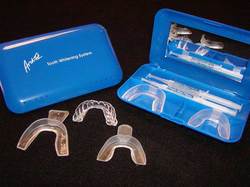
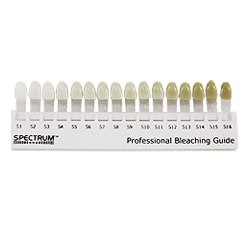
Bleaching is a procedure which involves lightening of the colour of a tooth through the application of a chemical agent to oxidize the organic pigmentation in the tooth.
Tooth bleaching today is based upon hydrogen peroxide as an active agent. Hydrogen peroxide may be applied directly or produced in a chemical reaction from sodium perborate or carbamide peroxide. Hydrogen peroxide acts as a strong oxidizing agent through the formation of free radicals, reactive oxygen molecules and hydrogen peroxide anions. These reactive molecules attack the long chained, dark coloured chromophore molecules and split them into smaller, less coloured and more diffusible molecules.
Carbamide peroxide also yields urea which is further decomposed to CO2 and ammonia. It is the high pH of ammonia which facilitates the bleaching procedure.
This can be explained by the fact that in basic solution, lower activation energy is required for formation of free radicals from hydrogen peroxide and the reaction rate is higher, resulting in improved yield rate compared with an acidic environment.
Ingredient of Bleaching Gels
- Carbamide Peroxide
- Hydrogen Peroxide and sodium hydroxide
- Sodium perborate
- Thickening agent-Carbopol or carboxy polymethylene
- Urea
- Surfactant
- Preservatives
- Vehicle-glycerine and dentifrice
- Flavours
- Fluoride and 3 % potassium nitrate
Carbamide Peroxide
It is a bifunctional derivative of carbonic acid. It is available as :
1) Home Bleaching
- 5% carbamide peroxide
- 10% carbamide peroxide
- 15% carbamide peroxide
- 20% carbamide peroxide
 2)In-office bleaching
- 35% solution or gel of carbamide peroxide
Hydrogen Peroxide
H2O2 breaks down to water and nascent oxygen. It also forms the free radical perhydroxyl (HO2) which is responsible for bleaching action.
Sodium Perborate
It comes as monohydrate, trihydrate or tetrahydrate. It contains 95% perborate, providing 10% available oxygen.
Thickening Agent
Carbopol (Carboxy polymethylene) : Addition of carbopol in bleaching gels results in :
- Slow release of oxygen
- Increased voscosity of bleaching material, which futher helps in longer retention of material in tray and need of less material
- Delayed effervescence-thicker products stay on the teeth for longer time to provide necessary time for the carbamide peroxide to diffuse into the tooth
- The slow diffusion intoenamel may also allow tooth to be bleached more effectively
Urea
It is added in bleaching solutions to : stabilize the H202, elevate the pH of the solution and anticariogenic effects.
Surfactant
It acts as a surface wetting agent which allows the hydrogen peroxide to pass across the gel tooth boundary.
Preservatives
Commonly used preservatives are phosphoric acids, citric acid or sodium stannate. They sequestrate metals such as Fe, Cu and Mg which accelerate breakdown of H2O2 ang give gels better durability and stability.
Vehicle
1. Glycerine : It is used to increase viscosity of preparation and ease of manipulation
2.Dentifrice
Flavors
They are added to improve patient acceptability
Indications of Home Bleaching
- Mild generalized staining for your teeth
- Age related discolourations
- Mild tetracycline stains
- Mild fluorosis
- Acquired superficial staining
- Stains from smoking tobacco
- Color changes related to pulpal trauma or necrosis
Contraindications
- Teeth with insufficient enamel for bleaching
- Teeth with deep and surface cracks and fracture lines
- Teeth with inadequate or defective restorations
- Discolouration in the adolescent patients with large pulp chamber
- Severe fluorosis and pitting hypoplasia
- Noncompliant patients
- Pregnant or lactating patients
- Teeth with large anterior restorations
- Severe tetracycline stains
- Fractured or malaligned teeth
- Teeth exhibiting extreme sensitivity to heat, cold or sweets
- Teeth with opaque white spots
- Suspected or confirmed bulimia bervosa
Advantages of Home Bleaching Techniques
- Simple method for patients to use
- Simple for dentists to monitor
- Less chair time and cost-effective
- You can bleach your own teeth at your own convenience
Disadvantages of Home Bleaching Techniques
- You need to comply to the technique
- Colour change is dependent on amount of time the trays are worn
- Chances of abuse by using excessive amount of bleach for too many hours per day.
Commonly used solution of nightguard bleaching :
- 10 % carbamide peroxide with or without carbopol
- 15% carbamide peroxide
- 1-10% hydrogen peroxide
Side effects of Home Bleaching
- Gingival irritation : Painful gums after a few days of wearing trays
- Soft Tissue irritation – From excessive wearing of trays or applying too much bleach to the trays
- Altered taste sensation – Metallic taste immediately after removing trays
- Tooth sensitivity – Most common side effects
When or how long to keep the trays in the mouth, depends on patients lifestyle preference and schedule. Wearing the tray during day time allows replenishment of the gel after 1-2 hours for maximum concentration. Overnight use causes decrease in loss of material due to decreased salivary flow at night and decreased occusal pressure. You can visit the dentist 1-2 weeks after wearing the tray so that the dentist can monitor your tooth and gums for better oral health. You can only whiten your teeth maximum up to 3-4 shades of your original tooth colour.